flocu/Getty Images
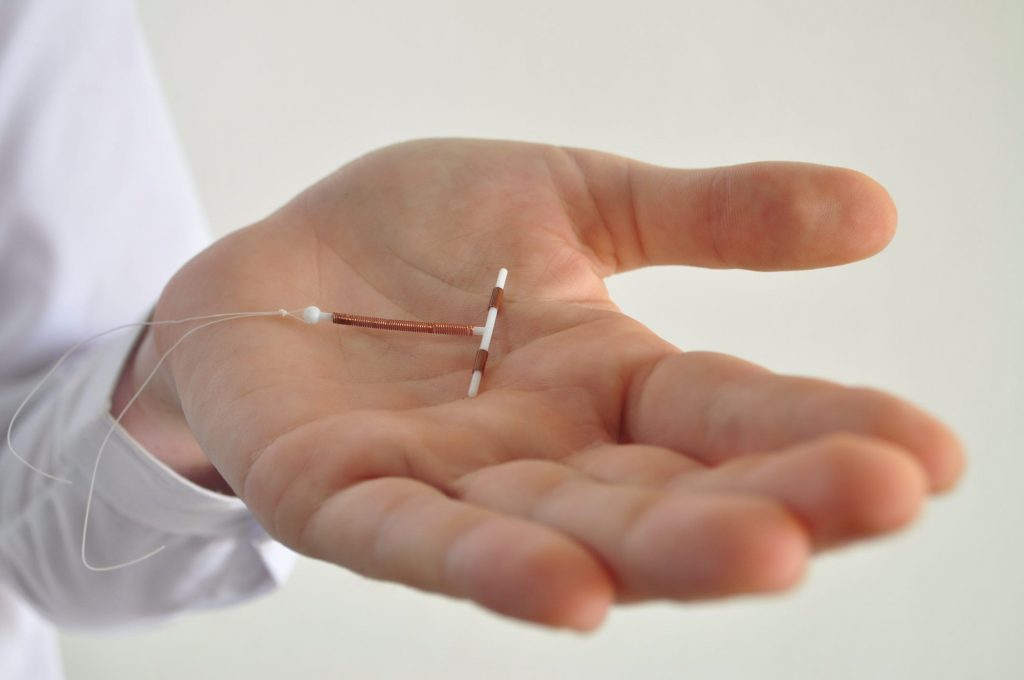
- A new copper IUD is smaller and more malleable than the only one FDA-approved in the US.
- Ongoing research is finding it's safe, effective, and well-tolerated in users.
- The design of copper IUDs hasn't been updated in 35 years, despite common side effects like heavy bleeding and cramping.
- Visit Insider's homepage for more stories.
Women in the US who want a birth control method that's long-lasting, reversible, and hormone-free only have one choice: the copper IUD, sold under the brand name Paragard.
The FDA-approved device is inserted into the uterus, where it can prevent pregnancy for up to 10 years by causing a reaction that's toxic to sperm and eggs. Its absence of hormones means users don't face risks like blood clots that can arise from popular hormonal options, like birth control pills.
But the design hasn't changed in 35 years, and side effects like severe menstrual cramps and heavy bleeding are common.
Now, researchers are studying a sleeker, more flexible IUD, called VeraCept, and finding it may offer people the benefits of Paragard with fewer of the drawbacks.
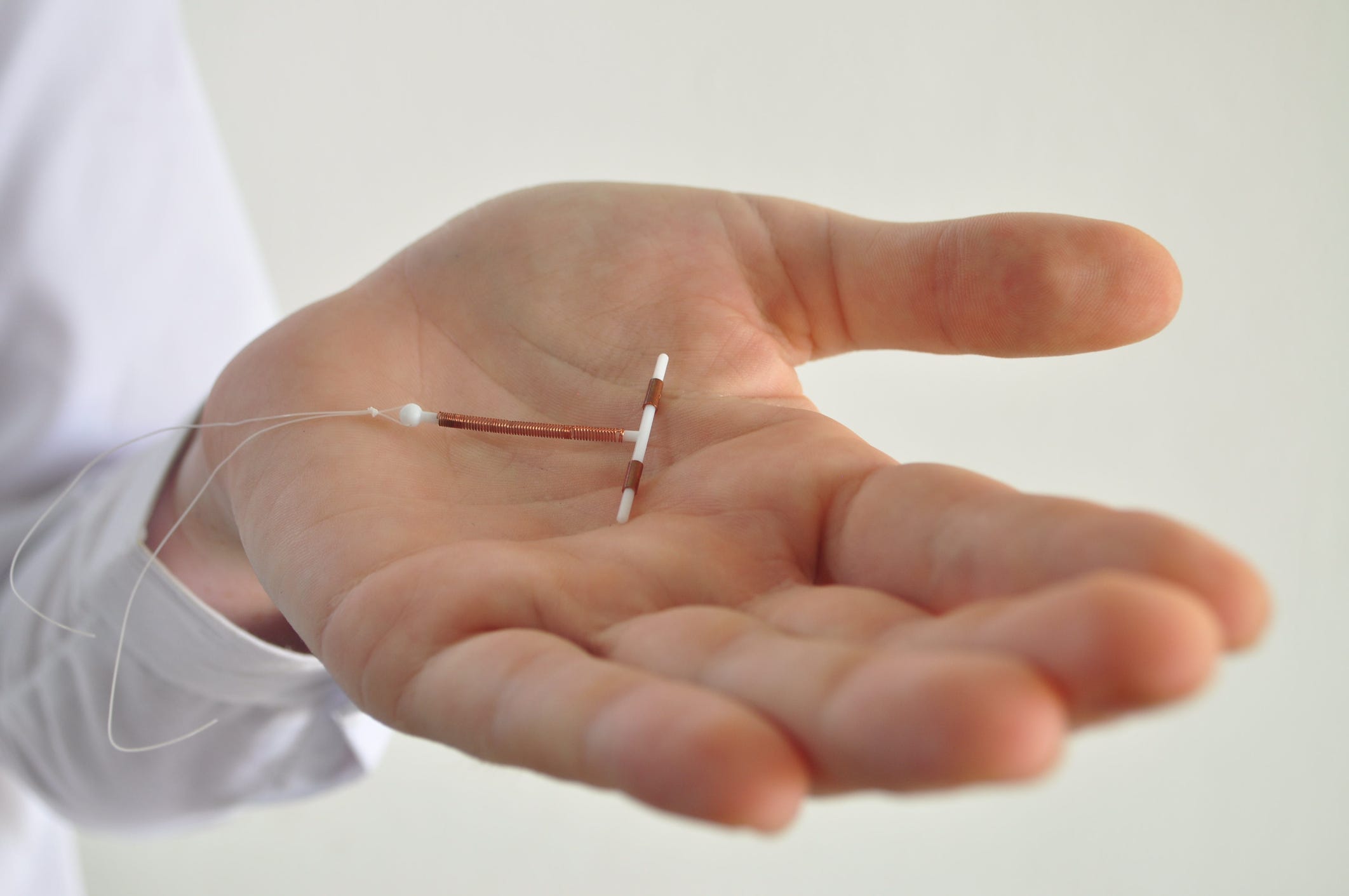
Courtesy of Dr. Mitchell Creinin
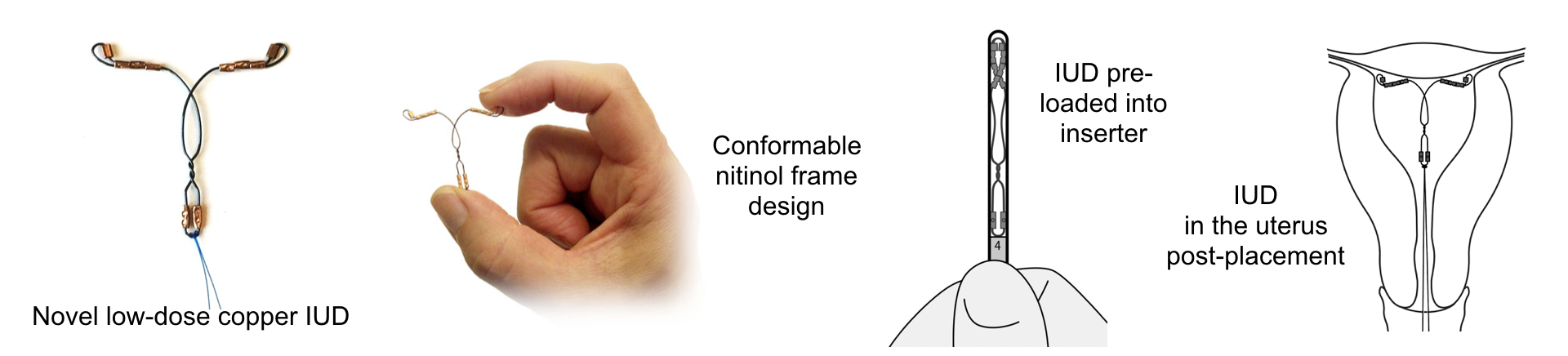
The device is smaller, with less copper and pre-cut strings
VeraCept, made by Sebela Pharmaceuticals, has less copper and is smaller than Paragard, and is made with a more malleable material in an attempt to lessen period cramps and bleeding. During normal contractions, the device "completely bends with the uterus," OB-GYN Dr. Mitchell D. Creinin of UC-Davis Health who's studying VeraCept, told Insider.
The flexible nickel titanium frame should also make insertion and removal more comfortable. Because it has "a forever memory," meaning it will always bounce back into its T-position in the uterus, Creinin said it can be "pre-loaded" into an insertion device for a smoother process.
The strings are pre-cut, too, eliminating another step during insertion.
An ongoing study suggests VeraCept is safe and effective
In the device's current study - a phase-3 clinical trial - Creinin and colleagues at multiple universities enrolled 1,619 people ages 17 to 45 across the US to use Veracept for five years. Most participants are under 36 years old and 60% have never had children before. About 74% are white, 19% are Hispanic, and 15% are Black.
Now, one year into the study, the researchers have found it to be highly effective.
Nine people, or one in about 180, became pregnant; two of them with ectopic pregnancies - a risk of IUD use. In comparison, about nine in 100 people on combination birth control pills get pregnant each year.
Results, which were presented at the American College of Obstetricians and Gynecologists' annual meeting earlier this year, also found the device to be quite safe. It was successfully inserted on first attempt in 97% of participants. Just 0.1% experienced perforations, or a puncture to the wall of the uterus, and 2.3% had expulsions. For context, about one in 20 IUD users currently experience compulsions.
People using VeraCept bled for about 11 days during their first period but that decreased to less than eight days by their third cycle. While about 95% of participants experienced at least one adverse event, most commonly heavy bleeding, only 14% of participants discontinued use because of it - a rate on par with other IUDs, OB-GYN Dr. Sarah Horvath wrote for Healio.
The study will continue for a few more years before Sebela pursues FDA approval.
"I think it's exciting that a company is trying to be very novel and say women shouldn't have to just say, 'This is the tradeoff I have to make," Creinin said. While it's too soon to know if they're successful, he said, "at least they're trying."
Dit artikel is oorspronkelijk verschenen op z24.nl